The Casting Project team: strength in numbers when fighting cancer
Date:
Changed on 29/11/2024
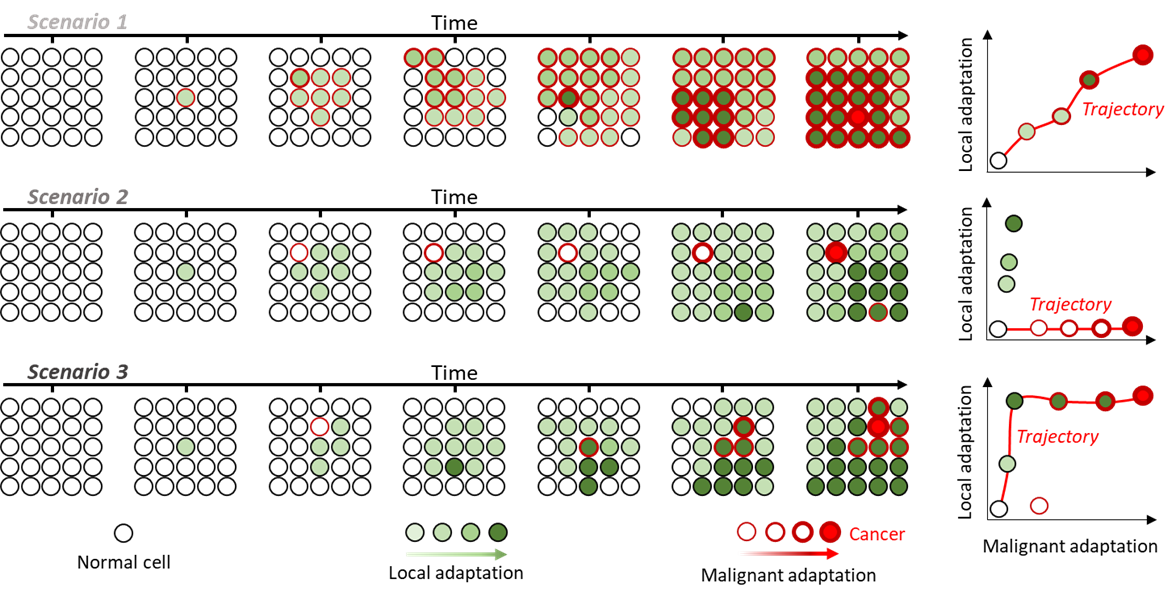
Uncoupling local and malignant adaptation: (Left) growth of a finite population over time under 3 different scenarios. Cells acquire alterations that differently affect their adaptation (green fill) and probability to become cancerous (red outline). (Right) Distribution of local and malignant adaptation scores for each clone and the trajectories leading from normal to cancer in red.
According to the WHO, Cancer kills 10 million people a year, and at least 100,000 researchers worldwide are studying this disease. So how do you approach this subject from a new perspective? That is the challenge taken up by the Casting (Cancer ecosystem dynAmicS, adapTation and modelING) project team, based in Lyon. Launched in June 2024 and supported by six supervisory institutions, including Inria (see box), it currently has 12 permanent staff.
The Casting project team adopts a doubly original approach. Firstly, a large part of its activity focuses on the evolution of healthy tissues into “preneoplasia” – precancerous conditions – and to the risk of these preneoplasia developing into the established malignant tumours that tend to monopolise research on every continent.
Casting's second specificity is that it brings together experts from different disciplines who usually only collaborate on a one-off basis or for the duration of a joint project.
“It's rare to combine mathematical modelling, computational biology, experimental biology and medicine in a single team," confirms Hélène Leman, an Inria researcher and co-director of Casting. We examine the same subject from four complementary perspectives with consistent aims, data and methods, and by extending the analysis to the cellular level. This promises to produce major advances in our understanding of the mechanisms by which healthy tissue and tumours evolve.”
What progress has been made? First of all: an accurate estimate of the potential for malignant transformation of preneoplasia. “For the cancers we are studying, those of the upper aerodigestive tract that may or may not be exposed to tobacco, there is currently no way of quantifying this risk," explains Professor Pierre Saintigny, a research physician at the Léon-Bérard Cancer Centre in Lyon and co-director of the team. “We implement surveillance for all patients, even though only a minority of them will go on to develop cancer.”
A better understanding of the development of preneoplasia will also help to identify possible therapeutic targets. This will enable other researchers to develop treatments designed to prevent malignant progression, if this is deemed likely, and will improve the effectiveness of screening and monitoring policies for patients at risk
However, Casting's research does not completely neglect established cancerous tumours. The team is striving to understand why metastases that have been reduced and inactivated by chemotherapy “reawaken” months or years later.
“The treatment creates selective pressure, which some cells resist and then start developing again,” explains Pierre Saintigny. “A bit like a boxer who’s temporarily stunned by a punch and then manages to get back into the fight after a variable amount of time. We need to understand what happens at the cellular level in such cases, in order to improve the therapies applied to these relapses.”
In practice, how do the team members organise their collaboration? “The key word is dialogue," replies Hélène Leman, a mathematics researcher who travels to the Léon-Bérard centre on a regular basis to work alongside her clinical colleagues. “For my part, I ask my colleagues in biology and medicine lots of questions in order to design models that target the relevant phenomena, and I validate these models using the data they provide me with.”
At the same time, experts in each discipline conduct their own research. For example, specialists in computational biology use samples taken from patients to carry out very high-throughput sequencing and then recover all the genes that have undergone mutations due to cancer, as well as the messenger RNAs they express. All this is then represented in highly complex realistic models: “They must faithfully reflect the biological and biochemical phenomena that occur,” states Pierre Saintigny. “In this way, we can deduce the mechanisms by which healthy, preneoplastic and cancerous tissues evolve”.
Understanding what is at stake is another objective of mathematical modelling researchers. Through discussions with biologists, they determine the types of cells and interactions between cells that play a key role. These scientists work at different levels: the cellular level to represent mutations, the tumoural level to reproduce their variations in size, and so on. They also establish the links between these different levels.
“The challenge is to obtain a model that comes as close as possible to reality, to accurately estimate its margins of error and to derive usable results,” specifies Hélène Leman. “For example, what is the probability of recurrence of a highly specific tumour previously treated with chemotherapy, or the risk of a malignant transformation of a given type of preneoplasia?”
Finally, researchers carry out in vitro, in vivo and ex vivo experiments to observe how cells, tissues or animal models react to various therapeutic molecules: do they develop tolerance or resistance? How quickly do cell populations develop?
“Combining and coordinating all this expertise within a single team is a sheer intellectual delight,” rejoices Pierre Saintigny. “It's also a way of producing highly original publications. Our researchers have already written two papers, one on mathematical theory and the other on the selection pressure of malignant cells induced by treatments.”
Some may be surprised that AI is not included in Casting’s toolbox. “It is highly effective at predicting phenomena,” replies Hélène Leman, “but much less useful for understanding and describing them. Particularly as it uses models in black-box mode: we obtain results without necessarily knowing the reasoning behind them.” The team does possess AI expertise, however, which comes into play at a later stage, for complementary purposes or for conducting virtual comparisons of the effectiveness of different treatment sequences.